Fda Biontech Kinder
Experts from the Advisory panel to the US Food and Drug Administration FDA have voted in favor of the Pfizer-BioNtech COVID19 vaccine. The fast track status could potentially make Pfizer and BioNTechs vaccine candidates BNT162b1 and BNT162b2 eligible for the US.
Covid 19 Vaccine Will It Be Safe For Kids And Pregnant Women
The emergency use authorization allows.
Fda biontech kinder
. - At least 165 million people have received a COVID-19 vaccine through the FDAs emergency use approval but medical experts said they recognize the lack of full FDA approval may be holding some people back. FDA advisory panel endorses PfizerBioNTech Covid-19 vaccine. The Food and Drug Administration is planning to authorize full approval of the Pfizer-BioNTech two-dose COVID-19 vaccine on Monday according to The New York Times. On May 10 2021 the FDA expanded the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine to include adolescents 12 through 15 years of age.According to the report the FDA originally had planned to. FDA cautions against off-label use of PfizerBioNTech Covid-19 vaccine in younger children Off-label refers to an approved product being used in a way or in a patient that it wasnt necessarily approved for. Pfizer-BioNTech submitted its application this summer for full approval and its being fast-tracked because of the pandemic. They have recommended the agency to grant emergency use authorization EUA to the vaccine.
2 days agoThe US Food and Drug Administration is on track to give full approval to the Pfizer-BioNTech coronavirus vaccine on Monday. The US Food and Drug Administration on Monday granted full approval to the PfizerBioNTech Covid-19 vaccine for people age 16 and older. Stocks in German pharmaceutical company BioNTech were up almost 18 at market close on Wednesday reflecting optimism after a recent report said that the FDA hopes to approve the companys Covid. With a new surge of coronavirus infections ripping through much of the United States the Food and Drug Administration has accelerated its timetable to fully approve Pfizer-BioNTechs coronavirus vaccine aiming to complete the process by the start of next month people familiar with the.
The drug needs to be kept at minus 60 degrees Celsius. The European Medicines Agency approved on Friday the use of the Pfizer-BioNTech coronavirus vaccine for children aged 12 to 15 in what the drug. May 28 2021. Notably several countries including the US have already granted emergency use nods for the vaccine.
BNTX BioNTech today announced that two of the companies four investigational vaccine candidates from their BNT162 mRNA-based vaccine program. The WHO statement termed it as a positive step towards ensuring global access to the vaccine. BNTX BioNTech today announced that two of. The Pfizer vaccine was the first of three.
NEW YORK and MAINZ Germany July 13 2020 -- Pfizer Inc. It however noted that the Pfizer-BioNTech vaccine needs ultra-cold chain storage facilities. Food and Drug Administrations priority review under. A panel of outside experts on Thursday recommended the Food and Drug Administration issue an emergency use authorization to the Covid.
FDA grants full approval to Pfizer-BioNTech COVID vaccine. It occurs generally with some medicine equivalent to when a chemotherapy accredited for one sort of most cancers is. PFE and BioNTech SE Nasdaq. Food and Drug Administration FDA but has been authorized for emergency use by FDA under an Emergency Use Authorization EUA to prevent Coronavirus Disease 2019 COVID-19 caused by severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 for use in.
FDA amended the EUA issued on Dec. It happens commonly with some drugs such as when a chemotherapy approved for one type of cancer is used to treat a different type. The new target is. The FDAs expansion of the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine to include adolescents 12 through 15 years of age is a significant step in the fight against.
11 2020 for. PFE and BioNTech SE Nasdaq. The Food and Drug Administration FDA granted full approval to Pfizer-BioNTechs COVID-19 vaccine for people 16 and older on Monday making it the first of the three vaccines being administered in the United States to move beyond emergency use authorization. This is the first coronavirus vaccine approved by the FDA and is expected to open the door to more vaccine mandates.
That answer isnt clear although the New York Times reports that their timetable is now clearer. Today FDA expanded the EUA for the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID19 to include adolescents 12-15 years of age. FDA cautions towards off-label use of PfizerBioNTech Covid-19 vaccine in youthful kids Off-label refers to an accredited product being utilized in a manner or in a affected person that it wasnt essentially accredited for. The Pfizer-BioNTech COVID-19 Vaccine has not been approved or licensed by the US.
Israel May Begin Vaccinating 12 To 15 Year Olds By End Of May The Times Of Israel

Pfizer Submits Initial Trial Data To Fda For Covid 19 Vaccine Booster T Gate

Usa Zulassung Fur Impfstoff Von Biontech Fur Kinder Ab Zwolf Jahren Zm Online

Biontech Usa Geben Corona Impfstoff Fur Kinder Und Jugendliche Frei Manager Magazin
Usa Lassen Biontech Pfizer Impfstoff Ab Zwolf Jahren Zu

Nutella Maker Tells Fda We Re Not Just For Dessert Anymore The Boston Globe

For Many Covid 19 Vaccines Come With A Side Of Side Effects The Verge
/cdn.vox-cdn.com/uploads/chorus_asset/file/22434520/1232163164.jpg)
For Many Covid 19 Vaccines Come With A Side Of Side Effects The Verge
Covid 19 Impfstoff Von Biontech Pfizer Fda Pruft Zulassung Von Comirnaty Fur Jugendliche

Pfizer Covid Vaccine Fda Clears Use In Kids Ages 12 To 15

Pfizer Covid Vaccine Fda Clears Use In Kids Ages 12 To 15
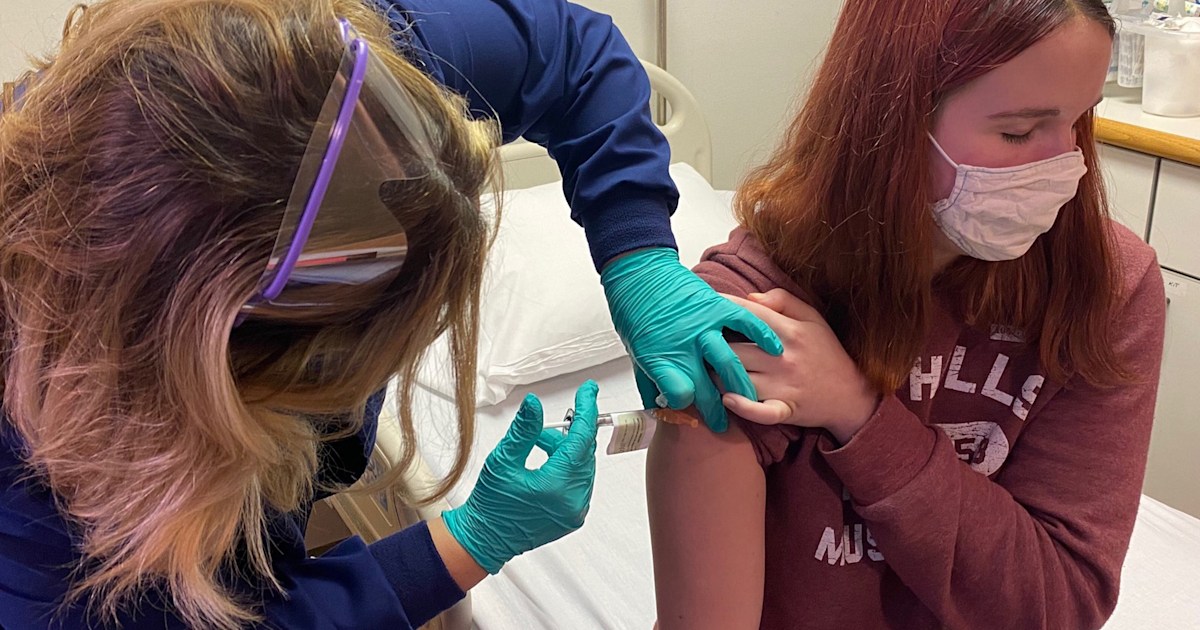
Covid 19 Vaccine Will It Be Safe For Kids And Pregnant Women
Food and Drug Administrations priority review under. The WHO statement termed it as a positive step towards ensuring global access to the vaccine.

Usa Zulassung Fur Impfstoff Von Biontech Fur Kinder Ab Zwolf Jahren Zm Online
The FDAs expansion of the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine to include adolescents 12 through 15 years of age is a significant step in the fight against.

Fda biontech kinder
. A panel of outside experts on Thursday recommended the Food and Drug Administration issue an emergency use authorization to the Covid. - At least 165 million people have received a COVID-19 vaccine through the FDAs emergency use approval but medical experts said they recognize the lack of full FDA approval may be holding some people back. Food and Drug Administration FDA but has been authorized for emergency use by FDA under an Emergency Use Authorization EUA to prevent Coronavirus Disease 2019 COVID-19 caused by severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 for use in. May 28 2021.FDA grants full approval to Pfizer-BioNTech COVID vaccine. It occurs generally with some medicine equivalent to when a chemotherapy accredited for one sort of most cancers is. That answer isnt clear although the New York Times reports that their timetable is now clearer. Notably several countries including the US have already granted emergency use nods for the vaccine.
Today FDA expanded the EUA for the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID19 to include adolescents 12-15 years of age. On May 10 2021 the FDA expanded the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine to include adolescents 12 through 15 years of age. The US Food and Drug Administration on Monday granted full approval to the PfizerBioNTech Covid-19 vaccine for people age 16 and older. Pfizer-BioNTech submitted its application this summer for full approval and its being fast-tracked because of the pandemic.
The Food and Drug Administration FDA granted full approval to Pfizer-BioNTechs COVID-19 vaccine for people 16 and older on Monday making it the first of the three vaccines being administered in the United States to move beyond emergency use authorization. It happens commonly with some drugs such as when a chemotherapy approved for one type of cancer is used to treat a different type. The Pfizer-BioNTech COVID-19 Vaccine has not been approved or licensed by the US. PFE and BioNTech SE Nasdaq.
The new target is. The Pfizer vaccine was the first of three. The European Medicines Agency approved on Friday the use of the Pfizer-BioNTech coronavirus vaccine for children aged 12 to 15 in what the drug. FDA amended the EUA issued on Dec.
FDA cautions towards off-label use of PfizerBioNTech Covid-19 vaccine in youthful kids Off-label refers to an accredited product being utilized in a manner or in a affected person that it wasnt essentially accredited for. With a new surge of coronavirus infections ripping through much of the United States the Food and Drug Administration has accelerated its timetable to fully approve Pfizer-BioNTechs coronavirus vaccine aiming to complete the process by the start of next month people familiar with the. PFE and BioNTech SE Nasdaq. According to the report the FDA originally had planned to.
BNTX BioNTech today announced that two of. The Food and Drug Administration is planning to authorize full approval of the Pfizer-BioNTech two-dose COVID-19 vaccine on Monday according to The New York Times. 11 2020 for. It however noted that the Pfizer-BioNTech vaccine needs ultra-cold chain storage facilities.
FDA advisory panel endorses PfizerBioNTech Covid-19 vaccine. This is the first coronavirus vaccine approved by the FDA and is expected to open the door to more vaccine mandates. FDA cautions against off-label use of PfizerBioNTech Covid-19 vaccine in younger children Off-label refers to an approved product being used in a way or in a patient that it wasnt necessarily approved for. 2 days agoThe US Food and Drug Administration is on track to give full approval to the Pfizer-BioNTech coronavirus vaccine on Monday.
Stocks in German pharmaceutical company BioNTech were up almost 18 at market close on Wednesday reflecting optimism after a recent report said that the FDA hopes to approve the companys Covid. The drug needs to be kept at minus 60 degrees Celsius. They have recommended the agency to grant emergency use authorization EUA to the vaccine. NEW YORK and MAINZ Germany July 13 2020 -- Pfizer Inc.
BNTX BioNTech today announced that two of the companies four investigational vaccine candidates from their BNT162 mRNA-based vaccine program.
/cdn.vox-cdn.com/uploads/chorus_asset/file/22434520/1232163164.jpg)
For Many Covid 19 Vaccines Come With A Side Of Side Effects The Verge

Pfizer Covid Vaccine Fda Clears Use In Kids Ages 12 To 15

Israel May Begin Vaccinating 12 To 15 Year Olds By End Of May The Times Of Israel
Usa Lassen Biontech Pfizer Impfstoff Ab Zwolf Jahren Zu

Pfizer Covid Vaccine Fda Clears Use In Kids Ages 12 To 15

Pfizer Submits Initial Trial Data To Fda For Covid 19 Vaccine Booster T Gate

Nutella Maker Tells Fda We Re Not Just For Dessert Anymore The Boston Globe

Covid 19 Vaccine Will It Be Safe For Kids And Pregnant Women

Covid 19 Vaccine Will It Be Safe For Kids And Pregnant Women

For Many Covid 19 Vaccines Come With A Side Of Side Effects The Verge

Covid 19 Impfstoff Von Biontech Pfizer Fda Pruft Zulassung Von Comirnaty Fur Jugendliche

Biontech Usa Geben Corona Impfstoff Fur Kinder Und Jugendliche Frei Manager Magazin







Posting Komentar untuk "Fda Biontech Kinder"